FOLLOWING THE SCIENCE AND EVOLUTION OF BEAUTY
How many times have we heard that collagen is important for our skin?
Collagen makes up 70% of the total protein found in the skin and is naturally synthesized by cells. It is responsible for preserving the structural integrity, elasticity, and firmness of the skin.
As we age, the body’s ability to produce collagen decreases, and its degradation increases, causing the structural integrity of our skin tissue to become more fragile and brittle. This leads to a weakening of the support of the extracellular matrix, resulting in a loss of skin firmness and volume, which in turn leads to the appearance of wrinkles, expression lines, and sagging.
Collagen: a powerful ally in slowing down skin aging.
Aging is inevitable as it is a natural process. Currently, in the market, various dietary supplements and dermocosmetics containing collagen are available, aiming to provide anti-aging benefits to the skin.
The most commonly used sources of collagen are derived from mammals and aquatic sources:
1st Generation Collagen – derived from mammals (cattle and pigs)
✅ Favorable biocompatibility;
✅ Wide accessibility;
❌ High molecular weight;
❌ Risk of transmitting diseases to humans;
❌ Not suitable for naturalists.
2nd Generation Collagen – Aquatic sources
✅ Low molecular weight;
✅ Low risk of transmitting diseases to humans;
✅ Abundant source;
❌ Unfavorable biocompatibility;
❌ Not suitable for naturalists.
With the advancement of technology, science is constantly seeking ways to make the signs of aging less evident by developing biocompatible, safe, and effective improvements and key ingredients that cater to all demographics.
Keeping up with the science and evolution of beauty, Bel Col introduces the Collagen Bio-Revolution: COLLAGEN 3.0 – THIRD GENERATION
Collagen 3.0 is a biomimetic collagen that corresponds to 100% of the DNA sequence of human collagen. It has high cutaneous permeability, poses no risk of disease transmission, and is produced in a laboratory, making it suitable for vegans as well.
Bio-Revolution of Collagen
BIOLAND HYUNDAI, a leader in technological raw materials in South Korea, conducted extensive research to create an innovative collagen that is biosynthesized in the laboratory through the cloning of human collagen DNA. This new collagen represents a significant advancement in the cosmetics industry and has been dubbed Collagen 3.0 – Skin to Skin Collagen!
Biosynthesis of Collagen through Human DNA
Among the various distinct subtypes of collagen distributed throughout our bodies, 90% of the total bodily collagen is classified as Type 1 Collagen.
Collagen molecules have a triple helix structure composed of three α polypeptide chains that wrap around a central axis. This conformation is achieved through the repetition of the amino acids glycine, proline, and hydroxyproline.
Before starting the biosynthesis of Collagen, the triple helix of the Type 1 Collagen molecule is divided, and the gene responsible for the most efficient anti-aging component is cut out. This gene is COL1A2.
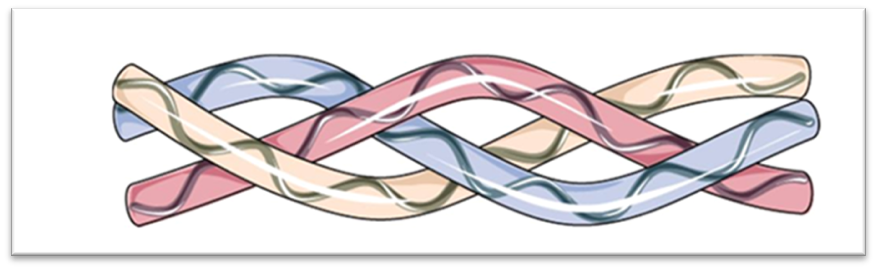
TRIPLE HELIX
Afterward, the DNA cloning process begins. Through this technique, it is possible to create many identical copies of human collagen using the COL1A2 gene, which is responsible for the most efficient anti-aging effect.
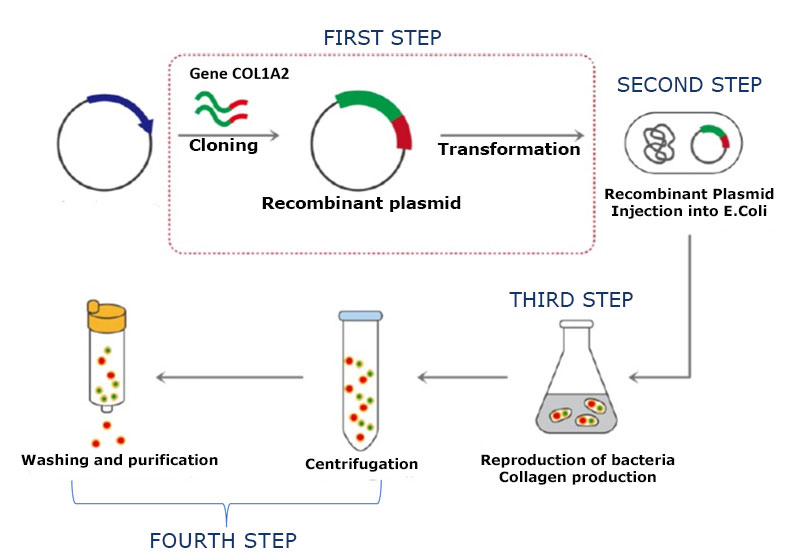
1st Step – The COL1A2 gene is introduced into a circular piece of DNA called a plasmid. This insertion is carried out with the help of enzymes that “cut and paste” the gene, forming a recombinant DNA molecule.
2nd Step – Next, the recombinant plasmid is inserted into bacteria. Bacteria containing the plasmid are selected and cultivated. As they reproduce, they replicate the plasmid and pass it on to their descendants, producing copies of the DNA it contains.
3rd Step – Batches of bacteria carrying the plasmid are cultivated and used as “factories” to produce the protein.
4th Step – Harvest the protein from the bacteria and purify it.
Representation of the process
The New Generation of Collagen
Since Collagen 3.0 is obtained through the replication of the most effective gene responsible for anti-aging, COL1A2, tests confirm that this bioactive has efficacy equal to or greater than TGF-b1 and EGF growth factors.
Furthermore, studies show that Collagen 3.0, due to its low molecular weight, exhibits greater permeability, making it a powerful anti-wrinkle and skin rejuvenating agent.
Efficacy tests confirm that Collagen 3.0 offers the following benefits:
100% biocompatible;
High permeability;
Induces elastin expression;
Increases Pro-collagen expression;
Has a potent anti-wrinkle action;
Reduces periorbital wrinkles.
Collagen is already great, and with all this innovation, it gets even better, doesn’t it?
Bel Col and Collagen
We are pioneers in the field of collagen and beauty, which is an integral part of our DNA: “Bel” from Beauty in Portuguese and “Col” from Collagen. In celebration of Bel Col’s 30th anniversary, we are once again pushing the boundaries of cosmetic science and making history by introducing the evolution of Collagen 3.0 into our products.
Bel Col 30 years younger – Skin-to-Skin Collagen! A dream of skin for a lifetime.
References:
- COSTA, Beatriz da; PORTO, Ana Lúcia; OLIVEIRA, Vagne; PORTO, Tatiana. “HIDROLIZADOS DE COLÁGENO, SEUS PRODUTOS E SUAS BIOATIVIDADES: uma mini-revisão” (COLLAGEN HYDROLYSATES, THEIR PRODUCTS, AND THEIR BIOACTIVITIES: a mini-review). Ciência, Tecnologia e Inovação: do campo à mesa, [S.L.], n. 15, p. 1-15, 2020. Instituto Internacional Despertando Vocações. http://dx.doi.org/10.31692/iciagro.2020.0202. Available at: https://doi.org/10.31692/ICIAGRO.2020.0202. Accessed on August 22, 2023.
- Roman JA, Sgarbieri VC. “Caracterização físico-química do isolado protéico de soro de leite e gelatina de origem bovina” (Physicochemical characterization of whey protein isolate and bovine gelatin). Braz J Food Technol [Internet] 2007 [accessed on January 19, 2014]; 10(2):137-43. Available at: http://www. sncsalvador.com.br/artigos/caracterizaco-fisico-quimicaisolado-proteico-soro-de-leite-gelatina-bovina.pdf
- ADAMIAK, K.; SIONKOWSKA, A. “Current methods of collagen cross-linking: Review.” International Journal of Biological Macromolecules, v. 161, p. 550-560, 2020.
